Ingredients
一个浅碗里
A candle - ideally a night light style one
A large glass or jar
Instructions
Float a candle in a shallow bowl of water.
Light the candle, leave it to start burning well
Place a large glass over the candle and see what happens
Result
You should find that after a few seconds the candle goes out.
Then the water level begins to rise
Explanation
When you burn a candle you are reacting oxygen from the air with carbon and hydrogen in the candle. This will reduce the amount of oxygen in the glass, but it will also increase the amount of carbon-dioxide in the glass.

Overall 3 molecules of oxygen will produce two of water which condense and two of carbon-dioxide. So the volume of gas should reduce by 1/3 of the oxygen burnt. Oxygen only makes up 20% of the air, and a candle will stop burning when the level of oxygen drops to about 15%, so the gas should shrink by about 1/3 of 5% so about 1-2%. This is probably too small an effect for you to notice.
What is actually going on?
Most of the effect is to do with the temperature of the gasses. As the candle burns it produces a plume of hot gasses rising above it., when you put the glass over it traps these hot gasses.
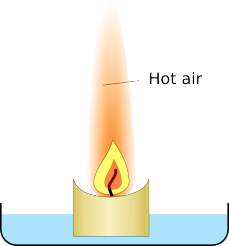 |
 |
| There is a plume of hot air and combustion products above a candle | The glass traps the hot gasses |
The glass slowly fills up with the hot air which takes up more space than cold air so some air escapes around the edges of the glass. Eventually the candle has used enough oxygen to stop itself burning, and it goes out.
The air then starts to cool, and as gasses cool they shrink so it pulls water into the glass producing a much larger effect than just the loss of oxygen.
 |
 |
| The glass fills with hot air with reduced oxygen levels and puts out the candle | The air then cools and shrinks pulling water into the glass |









Comments
Add a comment