When The Drugs Don't Work...
Antibiotics are chemicals that kill bacteria but leave us unharmed. However, bacteria are evolving so that our drugs no longer kill them. If this trend continues, the treatable are going to become untreatable... How serious would this scenario be, though? We'll be putting the problem under the microscope this week. Plus in the news, the UK's new Snooper's Charter, the man modelling vascular diseases in a dish, and what happens in your brain when you talk to God...
In this episode
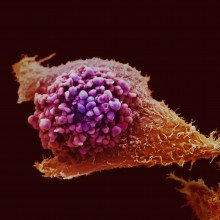
00:48 - New biomarker for prostate cancer found
New biomarker for prostate cancer found
with Assistant Professor Daniel Thorek, Johns Hopkins
First up - prostate cancer. About 50,000 cases of prostate cancer are diagnosed in British men each year. That's more than 10 times the number of cases of cervical cancer, and three times higher than the cervical cancer mortality rate. So why is there a screening programme for cervical cancer but not one for prostate cancer? Part of the reason is that there are some very effective markers for cervical disease, which make it relatively easy to discriminate between healthy people and someone with cancer. That hasn't been the case for prostate disease. Although now, scientists at Johns Hopkins in the US have developed a new antibody that can home in selectively on prostate tissue. And by coupling it to a labelling molecule, it can be used to highlight prostate cancers around the body and even deliver drugs to prostate cells. Daniel Thorek lead the study and spoke to Chris Smith about the discovery...
in British men each year. That's more than 10 times the number of cases of cervical cancer, and three times higher than the cervical cancer mortality rate. So why is there a screening programme for cervical cancer but not one for prostate cancer? Part of the reason is that there are some very effective markers for cervical disease, which make it relatively easy to discriminate between healthy people and someone with cancer. That hasn't been the case for prostate disease. Although now, scientists at Johns Hopkins in the US have developed a new antibody that can home in selectively on prostate tissue. And by coupling it to a labelling molecule, it can be used to highlight prostate cancers around the body and even deliver drugs to prostate cells. Daniel Thorek lead the study and spoke to Chris Smith about the discovery...
Daniel - One of the big problems that we have in prostate cancer patient management is how do we accurately detect the disease, is the disease localised to one spot or has it spread to many places? And then once we know what stage of the disease a patient has, what can we learn about that disease in order to best treat it? That's a longstanding problem in the field of prostate cancer because we know that the number of men who have this disease is very, very high. But how we treat these patients optimally is this longstanding question and this project was really aimed towards developing a new tool to detect disease, to characterise it, and to monitor it.
Chris - This is the concept of a biomarker, isn't it? Something you can measure which tells you about the disease, about the disease process and where the disease is.
Daniel - Right. So in prostate cancer we've been very fortunate because we had a very robust biomarker for quite a while, that is the PSA value, so the prostate specific antigen. This is a protein that is secreted from prostate cells and prostate cancer cells. As men age, we start to see a little bit more of this protein leaking into the blood and, if you have cancer, the number goes up even higher. The problem is using that number can be difficult to guide therapy. So, if the number of the PSA level in the blood is not very high, it can be difficult to discern if a patient has disease or not, or if a patient who has disease is responding to therapy or not.
Trying to improve on that test has been the main goal of this project. What we aim to do was to be able to be able to image individual lesions in the primary prostate to give an idea of the number of lesions and what molecularly was going on in those cells.
Chris - How?
Daniel - So the way we did this was we developed a novel antibody or a new antibody called 11B6. And the antibody targets a very specific portion of a protein produced by prostate cancer cells and by the prostate, and this protein is called HK2. We can then take that antibody, we can radio label it or we can put a fluorescent dye on it and we can inject that into either animals or, hopefully in the near future, into patients.
Chris - Now why did you choose that particular marker in the prostate to make this antibody that should recognise - why go down that path?
Daniel - That's a great question. So finding good biomarkers is really the difficult thing. We know that many cancer cells have aberrant expression of certain genes and, ultimately, of certain proteins. But many cells that are cancerous share all of the markers as healthy normal cells and so being able to select diseased cells specifically is a very difficult challenge. In the case of the prostate though we were a little bit lucky. So HK2, the protein that we're targeting with the 11B6 antibody is very similar to PSA, so it's specifically and only produced in men in the prostate and prostate derived tissues. So the only time you have prostate derived tissues is when you have prostate cancer, so here we have a protein that is specifically expressed by the organ that we're targeting and it's not expressed in any other tissues.
Chris - But that doesn't mean you can discriminate between a healthy cell and a cancer cell, they will both have this marker on because they're both prostate cells?
Daniel - That's absolutely correct. But in the case of prostate cancer we have a specialised case where any prostate tissue should be removed if a patient is at high risk of developing aggressive disease. And so, by using an antibody with an imaging agent tagged onto it, this 11BC antibody, we were able to target both healthy primary prostate tissue and diseased tissue. We get really the full gamut so we're able to really discern both malignantly derived tissue and healthy tissue, both of which we want to be able to characterise and hopefully remove.
Chris - Now will this work, that's the critical question, isn't it? Because you've done this in animals so far, have you got now a way to translate this to human patients to see if a) the antibody is safe, and b) if it does what it says on the tin - will it work in a human the same way it works in your experimental animals?
丹尼尔-它总是难以预测。我们're very confident that, in this case, we may have a really robust imagining agent to detect and characterise cancer in man. What's typically done is we take human cancer cells and put them into immuno-deprived mice, so those are mice that don't really have a functioning immune system which can allow transplanted human disease to grow in them. So that's usually how imagining agents are developed.
What we've done in these studies is we've used those models, but we've also evaluated models that look at disease in the bone. Prostate and breast cancer are very well known to metastasise to the bone, and imagining in the bone is very difficult - our antibody works there. We've also developed genetically engineered mouse models of disease. They have a fully intact immune system but they spontaneously develop cancer and so we can also use the antibody there. And finally, we've already initiated non-human primate studies, so we've done some toxicology in monkeys when we administer the antibody we've not seen any deleterious effects.

06:56 - Snooping on the Snooper's Charter
Snooping on the Snooper's Charter
with Peter Cowley, Tech Investor
In the last week, the UK Government's Investigatory Act became Law. Known as the 'Snooper's Charter' the law requires internet service providers to keep a record of all internet browsing history. The justification for the act is that it will help intelligence services counter terrorism, but critics point out that the information will also be shared with intelligence agencies but also government departments as diverse as HM revenue and Customs and the Department of Transport. Tech investor Peter Cowley joined Graihagh Jackson in the studio...
the 'Snooper's Charter' the law requires internet service providers to keep a record of all internet browsing history. The justification for the act is that it will help intelligence services counter terrorism, but critics point out that the information will also be shared with intelligence agencies but also government departments as diverse as HM revenue and Customs and the Department of Transport. Tech investor Peter Cowley joined Graihagh Jackson in the studio...
Peter - They're storing the record of where you browse, so where you've looked at. They would, for instance, store the fact that you've been on the BBC website and how long you've been there. What they're not storing, at the moment anyway who knows later, is which pages you looked at. Whether you've gone on the technology page, the politics page etc., and all the internet service providers, the ones we log on to, will store that for up to a year.
The justification is that by having somebody who's committed a crime or is likely to commit a crime, looking at their browsing history will give you an idea of what they've been looking at and, therefore, what they might have been researching on. So, an extreme example would be bomb making. You'd go onto a site which is only about bomb making, that would then make the connection there for the security forces.
Graihagh - Surely a' needle in a haystack' kind of scenario. I'm thinking of how many web pages I look at in a week and then times that by the population of the U.K. - a huge number of people and data there. Do you think this is a step too far?
Peter - Definitely a step too far. The actual amount of data, if they can work out what your ISP was, they can at least go to that ISP and work out what you've connected on.
Graihagh - Sorry - ISP?
Peter - Internet Service Provider - that's the person you're actually connect to. There will be a vast amount of data. The thought of actually looking through all this data for a whole year, as you say, how many zeros do you want to go to?
Graihagh - Mind Boggling!
Peter - Yeah. So to actually look through all the data will be almost impossible, but I think they will be targeting somebody at that point.
Graihagh——你可能认为,实际上,everything is hackable these days, nothing's really safe. So how safe do you think this is going to be with the internet service providers?
Peter - Very unsafe! If it's regarded by somebody who wants to hack it as a very valuable source of data, which it probably will be.
Graihagh - Why would it be valuable?
Peter - Well, it would be valuable because imagine the situation where you're browsing on say some medical condition that you don't want other people to know about. That could be used in a way that you would lose out on.
Graihagh - Blackmail maybe?
Peter - Blackmail, exactly. I was thinking also your medical insurance company might be. I'm sure it wouldn't go that far. It's data that you want to keep private to yourself. Your religion, there's a number of other things you could be looking at looking at.
Graihagh - And that's not safe - this information won't be safe in your opinion?
Peter - Not at all! All these databases are hacked. But you've mentioned a step too far. At the moment we of course, in principle, trust our governments, but imagine once this things in place at what point can you continue to trust the government. I mean, there has been change in government in the States, hasn't there?
Graihagh - Well speaking of the States, you know the UK is one of the most surveyed counties in the world. What's the stance elsewhere, in America say?
Peter - In America, and Australia, and Canada, and New Zealand, for instance, they have a similar system in that they can store this data but they do need a warrant before it can actually be accessed. At the moment this is set up so it just needs a senior manager to say they can look at someone's data.
Graihagh - A senior manager! That's it?
Peter - A senior manager, yes. An inspector rank within the police, for instance.
Graihagh - Wow!
Peter - And another thing, if you want to hide this you can easily. You can hide behind VPNs, which is a virtual private network. Behind whatsapp, for instance, has got it's own encoding or encrypting. And, of course, the people who want to hide - will. They'll put these systems in place. An HTTPS, which is a secure site used for banking, that data will not be accessible or readable. So it will be the innocent people that will have their data stored and the guilty will have worked a way round it.

10:56 - Growing blood vessels in a dish
Growing blood vessels in a dish
with Dr Sanjay Sinha, Addenbrooke's Hospital and Shona Cobb
A new technique that enables doctors to recreate a patient's disease in a dish and it has been pioneered at Cambridge University where cardiologist Sanjay Sinha is working on disorders of blood vessels and he told Chris Smith about his work...
and it has been pioneered at Cambridge University where cardiologist Sanjay Sinha is working on disorders of blood vessels and he told Chris Smith about his work...
Sanjay - We take advantage of making stem cells from patients who have diseases. We can actually take a skin biopsy from these patients and using those skin cells turn them into stem cells, and then we can use these stem cells to generate blood vessel tissues. Basically smooth muscles cells are the ones we primarily study and these are the cells that form the walls of most blood vessels.
我们started off looking at aortic aneurysms and we focused on a condition called Marfan syndrome. This is a genetic disorder, so it runs in families. People who have Marfan's have overgrowth of their long bones, hyperextensible joints and, in fact, it's speculated that Abraham Lincoln might actually have had Marfan syndrome. That's the sort of typical appearance of somebody.
But the biggest problem that these people face is the development of aortic aneurysms. The aorta is the biggest blood vessel in the body. It's like a large hosepipe coming out of your heart and if that ruptures or tears, it's absolutely catastrophic for the patient.
Chris - What fraction of people who are affected by Marfan's actually get that problem?
Sanjay - Well a large percentage of them actually. But the majority of them will have increasing aortic size. And, at the moment, the only treatment that's effective for preventing rupture or tearing of these vessels is surgery. There's no effective medical treatment at the moment and that's what we'd like to do is to come up with a new treatment that can actually prevent aneurysms and prevent such drastic surgery from being needed.
Chris - And so using your technique, does that mean we'll gain potentially more insight into why the disease does what it does?
Sanjay - That's exactly what we want to do. The smooth muscles cells that we make from the stem cells of patients, we can see the same abnormalities in the culture dish that we see in a patient's aorta. So the abnormalities were to do with the breakdown of extracellular matrix connective tissues, increased stiffness of the smooth muscle cells, the increasing smooth muscle cell death, we see all that in our culture dish as well.
So we can use this system now to study the molecular mechanisms - what might be causing those abnormalities? We can also use it as a screen to say what new medicines or drugs can we use to try and prevent those abnormalities.
Chris - Can we take a look at the laboratory where you're making these diseased blood vessels in dishes?
Sanjay - Off course - let's go... We keep the cells in this incubator at 37 degrees. So if you have a look down here, you'll see round patches of cells. Now these are stem cells that we have generated from patients with Marfan syndrome.
Chris - I'm looking down the microscope and I'm seeing blobs. They look like little islands if I was to look at an archipelago of islands in the ocean. What am I seeing?
Sanjay - You're seeing stem cell colonies - these are collections of cells. As they grow bigger we can then treat them with cocktails of growth factors and then these will eventually make smooth muscle cells.
Chris - How do you know when they've turned into a muscle cell?
Sanjay - They change their appearance. They'll start to look like long spindles, so very different in appearance to what we see here, in fact, much bigger. And, in fact, if you then stimulate them you can actually see the muscles contract.
Chris - Goodness! And once you've got them into muscle, how do you then sort of start asking questions of them about the disease they would be harbouring were they in a patient?
Sanjay - We can do studies looking at the different proteins that are expressed. We can look at changes in the DNA, whether the cells are alive or whether they're dying. And using all of these approaches we can see whether the cells are healthy as you might expect in a healthy vessel, or whether they're reproducing the abnormalities that we see in patients who have Marfan syndrome.
Chris - Sanjay Sinha. But what does a discovery like this mean for a person who actually has the condition?
Shona - My name is Shona Cobb and I have Marfan syndrome, and I write a blog called Shona Louise. When I was growing up I went to Great Ormond Street a lot and to me that was just sort of a fun day out. That's how I associated it.
Chris - This is the hospital for kids in London?
Shona - Yeah. And then as I grew older I leant more about it. I learnt about our family history and what happened with my uncle and my grandad who both suffered aortic dissections when they were in their thirties, so I learnt how important it was. And then this year it became more central in my life as we discovered that my measurements were progressing towards the point where they would operate.
Chris - So this is the main blood vessel coming out of the heart in you is stretching now?
Shona - Yeah. My aortic root is getting quite close to the point where they usually say it's time to step in.
Chris - And what will that mean?
Shona - It will mean major open heart surgery. Having a whole part of my aorta replaced to remove the part that has widened to stop it from bursting. So it will be quite extensive surgery, especially for someone of a young age.
Chris - How old are you?
Shona - I'm nineteen.
Chris - Now when you hear about a piece of research like Sanjay has done here being reported about the condition that is very close to your heart - no pun intended - what goes through your mind?
Shona - It's amazing. For someone like me it's going to be up there as a day I will remember for the rest of my life. And I'm really not exaggerating because this is big news for me and many others. We have a rare condition that we often go to the doctors and they don't know what it is or they don't know enough about it and they sit there and google it in front of us. So to have someone say that they've spend time and energy looking into our condition and they've actually found something which is a big step forward, you know, it's amazing.
Chris - What about the point though, that for many people with these sorts of conditions, it will be too late to stop them from getting the kinds of complications like you have got?
Shona - Yeah, I've been thinking about that a lot because, obviously, it is too late for me and it's too late for some of my family as well, but I think it brings hope for the next generations to come. And there's a lot of people who have children who have only just been diagnosed with it or having babies and wondering if they have it. To be able to have someone say, you know things might be different for them is what we want.
Chris - Shona Cobb. So what new avenues has this ability to reproduce a patient's disease in a dish delivered to us. Sanjay Sinha again...
桑杰,与马方氏大多数人,the gene is fibrillin-1, and mutations in of fibrillin-1. What happens is there's a lot less fibrillin-1 in the aorta and there's also degradation and breakdown of other connective tissues that leads to weakening of the vessel wall and there's also loss of smooth muscle cells. And what our work has shown is that there's another pathway that we've identified mediated through a protein called P38 that's important for smooth muscle cell death. And P38 is overactive in Marfan sydrome, and if we actually block it we can rescue this smooth muscle cell death.
Chris - And that's what this new approach is showing you?
Sanjay - That's what the new approach has shown us. And I think it's really important and actually really exciting because there are medicines available that drug companies are developing to block P38. These have been used in clinical trials in other patients with other conditions so we know these drugs are safe. They've never been tried in patients with Marfan syndrome, and I think this is really exciting because our study suggests that we could use these drugs that we know are relatively safe. So very close, I think, to clinical trials using this approach.

19:55 - The latest cyber crime: Ransomware
The latest cyber crime: Ransomware
with Jonathan Bowers, UKFast
As our lives increasingly shift online and we rely more and more on computers and cybercrime and hacking are on the increase. And recently there has been a big surge in reports of private individuals and businesses having their computers hijacked by a particularly malicious process dubbed ransomware. This is where users are locked out of their machines and all their data is irreversibly encrypted unless they pay a large fee to the hacker who then sends an unlock key to let them back in. Chris Smith spoke to Johnathan Bowers from UKFast who have been monitoring the situation...
Jonathan - Ransomware is, essentially, an attack that locks people out of their computers and can occasionally even lock people out of whole business networks. The most usual way for ransomware to take hold is by somebody actually clicking on a link and downloading a virus. A piece of information that takes hold of, through encryption, the computer and doesn't allow you to gain access to it. Unfortunately, as in lots of cases with internet security, it's human error that lets people into something like a ransomware attack. The victim actually clicking on a link and downloading something that they shouldn't to their computer.
Chris - But what will the form take of something turning up? Will it be an email that looks innocuous or will it be obviously something that they shouldn't be clicking on?
Jonathan - Quite often it will be an email and nowadays it's an email that will look fairly innocuous. The sophisticated methods are really improving. They're becoming much more targeted and, essentially, people are finding out more about your organisation even, occasionally, sending an email that is actually spoofing the email address of somebody else. Perhaps the finance director within your company asking you to download a piece of information.
Chris - What would be the average experience of the person that this happens to? Just take us through the journey that got to them having a locked computer - what does it look like, what happens to them?
乔纳森,所以人会发生什么事situation is that they will download something that they think is fairly innocuous but very quickly, once that file executes on your computer, you then can't actually log in and gain access. Shortly after that, you'll receive information telling you that you would have to pay the ransom. It will tell you that your information has been encrypted and, unfortunately, because of the sophistication of encryption techniques on computers nowadays it's nigh on impossible for someone to actually try and break that encryption and manage to rescue themselves from the situation.
Chris - And when you say ransom - what's the ransom that's usually asked?
Jonathan - The ransom will usually be in the form of something like bitcoin because it's easier and easier to mask where that currency is going or coming from. It makes it even harder to try and track down who's doing this kind of thing.
Chris - How much ransom are we talking about on average?
Jonathan - Well, the ransom itself is increasing. Around a year ago you might have been looking at a ransom of say £3,000 in order to get your whole business back up and running again. But these ransoms have actually increased in the last 12 months by about 135 percent and will carry on increasing as well as people start actually paying them.
Chris - Do we know who is doing this?
Jonathan - It's very difficult to say who's actually doing it. We know a lot of people are doing it and we know that the barriers to entry have come down dramatically. And we'll find that a lot of people that are doing it are probably doing it for somebody else and essentially it will be an area of cybercrime where script kiddies are playing a major part. They may even not know who they are doing it for necessarily, but they'll be getting a cut of the money that they make.
Chris - Do we know where these people are based?
Jonathan - We don't know where these people are based and the sophistication of cybercrime means that it's extremely hard to trace that kind of thing.
Chris - Based on your experience as a UK hosting company, what do you think the incidences of this are - are you seeing an increased trend?
Jonathan - In 2015 we had about 20 cases. In the last three months of 2016, we've had over 30 cases. I guess that would show how much this is increasing.
Chris - Can you unlock that data for those people or is it literally a case of they have to pay up?
Jonathan - In the vast majority of cases with us, luckily that client will have taken backup solution with us and, therefore, what we would be more likely to advise is that they roll back to the latest backup, and that will allow us to get the information back on a fresh machine and get them going again. It means they can actually refuse to pay the ransom and keep moving.
Chris - That would be your number one piece of advice would it to a) don't open dodgy attachments if you can avoid it, but b) definitely have a backup?
Jonathan - The backup plan is absolutely crucial for people - making sure they've got a backup. But I would add to the first one there to a) because sometimes it's getting so sophisticated that people need to be actually educated on the types of things, perhaps within a business, they should and shouldn't open. They should know whether the finance director will ever actually send you an email asking you to download something.This can be put into inductions within businesses to make sure people are much more savvy about what they should and shouldn't download and I think that would then help overall to protect businesses.

25:03 - Sex, drugs and religion
Sex, drugs and religion
with Assistant Professor Jeffrey Anderson, University of Utah
According to new research from the University of Utah, finding God can feel a lot like sex, drugs and rock and roll. And this is because all of these hedonistic pursuits activate the same reward pathways in the brain as a religious experience. Graihagh Jackson caught up Jeffrey Anderson, who made the discovery...
like sex, drugs and rock and roll. And this is because all of these hedonistic pursuits activate the same reward pathways in the brain as a religious experience. Graihagh Jackson caught up Jeffrey Anderson, who made the discovery...
Jeffrey - Billions of people find meaning and make important decisions based on religious and spiritual experience, yet we know so very little about how the brain interacts with these experiences. And we set out to study a specific type of spiritual experience termed "feeling the spirit" in a group of devout Mormons. Perhaps the most striking finding to us was activation of the nucleus accumbens, and area that's been termed the brain's reward centre. So romantic love, parental love, winning at gambling, cocaine and methamphetamines, all of these types of experiences strongly activate the same region.
Graihagh - Is that surprising though because people enjoy being religious?
Jeffrey - Well, absolutely! I would think that to a believer, of course this is going to activate brain reward circuits. They're rewarding experiences so that shouldn't be surprising to us, yet this is the first study that's actually associated those types of circuits with religious experiences.
Graihagh - Jeffrey came to this conclusion by popping nineteen Mormons into an MRI scanner and got them to do a range of tasks from listening to religious speeches and quotes to...
Jeffrey - ...to personal prayer to audio-visual stimuli produced by the church that were designed to evoke the types of spiritual feelings that we set out to study.
Graihagh - And then, he got them to press a button when they felt the spirit.
Jeffrey - It was surprising to us how well we were able to recreate those feelings in the scanner; it's kind of a private place.
Graihagh - Mmm, that is surprising. I know what you mean about an MRI being a private place but it's also hugely noisy as well from what I remember.
Jeffrey——是的嘈杂的喧哗、人工、然而,somehow very conducive to these types of feelings. Many of the participants that we studied were in tears at the conclusion of the scan and they reported that these were very strong feelings that were very similar to what they had in their own religious practice or worship services.
Graihagh - Interestingly, the reward pathway in the brain lit up three seconds before the Mormons pressed that button. Their breathing deepened as well and their heart rate quickened. But is this going to be true of all faiths, not just Mormons?
Jeffrey - Well, it's a compelling hypothesis. We don't know exactly how this will differ from individual to individual, or from faith tradition to faith tradition. But there's a strong argument that there's a shared library of brain responses to religious and spiritual experiences, both adaptive and maladaptive types of religious experience.
Graihagh - I asked that because your study I think was on nineteen individuals, so it it really a big enough study to then be able to say well, you know it might be like this - is that not a bit of big jump?
Jeffrey - When you make a jump to other faith traditions, you know that's going to require more studies. We need to study other groups but the tools are mature. Religious neuroscience has the capability to answer questions that have been around for millenia.
Graihagh - Like what?
Jeffrey - Like what is it that the brain is doing when we feel these profound spiritual experiences?
Graihagh - For me, the big question was this. Given that feeling the spirit lights up the reward section of the brain, could it then compete with things like sex, drugs, and rock and roll? Could this explain why lots of religions ban such pleasures so worshipers remain faithful?
Jeffrey - Maybe those things are competitors to the rewards that induced by religious experience. It's so pervasive that religions have rules about sex reward, other pleasure inducers. And, on the other side, many religious traditions actually use these types of experiences to reinforce religious conviction like peyote or the effects of music, which is known to activate the same brain regions, social rewards and reinforcement.
Graihagh - What can we learn from this? It's interesting - sure. But could we use religion or even music to treat addicts, retrain those neural networks to get a high from something else that's less damaging?
Jeffrey - That's a really interesting idea. We know that people who are addicted to drugs and alcohol, many of the treatment strategies have used religion. And there may not be too far of a stretch to imagine why if it's a lot of the same circuitry that's involved. We don't typically talk about addiction in terms of religious experience or in terms of romantic love, but a lot of the same physiology is involved. There's withdrawals, there's dependency, there's powerful motivation of behaviour and a lot of it has to do with whether something is adaptive or maladaptive in your life.

30:48 - What's growing on your desk?
What's growing on your desk?
with Dr Adam Roberts, UCL
Antibiotics are chemicals that kill bacteria but leave us unharmed. However, 细菌是不断发展的,这样我们的药物是没有经度ger kill them. And if enough species of bacteria become resistant to sufficient numbers of our antibiotics, the treatable are going to become untreatable, and relatively simple infections, and also simple hospital procedures will become life-threatening. How serious would this scenario be? After all, antibiotics were only discovered a little under 100 years ago and we survived up until then, so surely we can do the same again? We'll be putting the problem under the microscope this week, starting with where antibiotics came from in the first place. Liam Messin went to see UCL microbiologist Adam Roberts...
细菌是不断发展的,这样我们的药物是没有经度ger kill them. And if enough species of bacteria become resistant to sufficient numbers of our antibiotics, the treatable are going to become untreatable, and relatively simple infections, and also simple hospital procedures will become life-threatening. How serious would this scenario be? After all, antibiotics were only discovered a little under 100 years ago and we survived up until then, so surely we can do the same again? We'll be putting the problem under the microscope this week, starting with where antibiotics came from in the first place. Liam Messin went to see UCL microbiologist Adam Roberts...
Adam - Antibiotic resistance is probably as old as the bacteria themselves. It's a tried and tested evolutionary strategy to produce these natural antibiotics which we use in medicine in order to give the bacteria that produced them a competitive advantage over their neighbours.
Liam - So the majority of antibiotics we're using were first made by a bacteria?
Adam - That's right, yeah. They're natural products which were discovered usually by analysing soil microbes - bacteria and fungi that live in the soil.
Liam - So we're talking on evolutionary timescales. What's the oldest known record we have of antibiotic resistance then?
Adam - I can recall a paper inSciencea few years ago where they looked at 30 thousand year old permafrost and the bacteria that they analysed in that sample had resistance genes in their genomes, which were pretty similar to the ones that we find in clinical isolates today.
Liam - How widespread are these antibiotic resistance genes - is it just limited to soil and permafrost? I mean, I'm in an office right now and my office doesn't have antibiotic resistance bacteria, does it?
Adam - I think it probably would and we could test for that quite easily, actually. If I sent you some swabs and you swabbed whatever you want to in your office, your desk maybe or your computer keyboard and then send those back, we could grow all of the isolates that we can from those swabs and then we can test for both antibiotic production against a range of indicator strains we have. And we can also test whether the isolates from your swabs are resistant to a whole suite of different antibiotics. So you'll have a swab-off.
Liam - Let the swab-off commence...
Graihagh - So shall we label this so we've got it right? So this one's my desk right?
Liam - Yes, Graihagh's desk.
Graihagh - Graihagh's desk. Ah, I'm nervous about what's going to be on here. Okay, swab number one done. Swab number two...
Liam - I should say my keyboards been used by a lot more people than just me.
Graihagh - Yeah, yeah, yeah. Right which bit do you reckon? The space bars probably used the most, right?
Liam - Yeah, the space bar, yeah. Because my keyboard was so disgusting, we took two samples to satisfy our curiosity.
Graihagh - Ah, there's black stuff on it!
利亚姆-啊,这可能只是因为我工作hard! All swabbed up - there was only one thing left to do. Who do you think is going to be the worst? Place your bets now!
Adam - We're in a class two lab this morning so we've got to put the lab coats on and follow all the rules because we work with some quite interesting pathogens.
Liam - Adam had taken our grubby swabs to see what he could grow on the equivalent of bacteria food, agar. And the results were, as expected, pretty grim!
Adam - You can see here that on samples two, three, there's quite a lot of different bacteria.
Liam - All three of the plates had grown a mass of furry stuff. One even looked like someone had sneezed on it. But, interestingly, no two swabs were alike despite the fact I swabbed the J key and the space bar of my keyboard. They're centimetres apart but entirely different bacterial colonies.
Adam - So it just shows that the microbial diversity centimetres away from one area will be different in another.
Liam - Adam then grows these colonies further, before putting them onto some more agar but this one contains antibiotics. So, if they grow here it means we have antibiotic resistant bacteria living on our keyboards!
Adam - And you can see clearly here from swab number four, so this is your second keyboard swab. This is the one, which there was a lot of bacterial cells on it, but it all seemed to be the same thing. So that's growing on ampicillin really well.
Liam - So there was antibiotic resistant bacteria on my keyboard?
Adam - Yeah. So it's growing on ampicillin, it's growing on tetracycline, it's growing on kanamycin and it's also growing on chloramphenicol.
Liam - So it's resistant to all those antibiotics?
Adam - All four, yeah. So it's a multidrug resistant bacterial isolate. It's resistant profile is quite surprising.
Liam - Oh, really? Surprising in a good way?
Adam - Well... I wouldn't have expected something to grow on all four of those antibiotics. They're all different chemicals and they all have different activities, so to find something that grows on them all is unusual.
Liam - Yikes! Where's the desk disinfectant? I fared pretty badly then, but what about Graihagh? Well, she didn't have any resistance but she did have bacteria that were killing off other bacterial nasties.
Adam - The second isolate is producing something, pumping it into the agar, and that's preventing theMicrococcusfrom growing.
Liam -Micrococcusis just a weak bacterium. It lives on our skin and does healthy people no harm. However, if Adam matched Graihagh's strain against something a little more lethal, how would it fare?
Adam - We've got a multidrug resistantE.coli, and you can see that the very small zone of inhibition.
Liam - So Graihagh's sample has something that appears to be killing multiple drug resistantE.coli?
Adam - That's correct, yes. So what we really want is something that can kill exactly this type of bug because that's what's causing problems in the clinic, so it's really encouraging we find something like that. So it just shows how easy it is to find antibiotics pretty much anywhere in the environment. Whether they're new and useful for medicine, obviously will take many years of study to find out but, if we don't look, we won't find them.
Chris - That was Adam Roberts there. And If you'd like to learn what's lurking where you live and work, you can get in touch with Adam via his website, SwabAndSend.co.uk... So can we get rich from your antibiotic-producingE.coli, Graihagh?
Graihagh - Well, great minds think alike because I put this to Liam, and sadly not. It may have been growing on my keyboard but because Adam has discovered it, it belongs to him.
Chris - So can we actually see what these bugs look like then?
Graihagh - Yeah, it's pretty grimy. I've got some photos of the petri dishes here in which we've grown. These first two are Liams - they sort of look like yellow lichen on a petri dish, don't they?
Chris - Yeah, lots of little blobs of bacterial colonies.
Graihagh——美味!然后在我的盘子我有mething similar, this yellow lichen stuff but then I've also got this weird milky substance at the top corner.
Chris - Some kind of mould isn't it, or something?
Graihagh - Yeah, it doesn't look particularly nice.
Chris - Swarming all over the place, nice!
Graihagh - But what's particularly interesting is this last plate that Liam and Adam discussed. And this is a plate with bacteria already on it and we put that little E.coli-killing strain that was on my keyboard and we grew it. And what's really interesting you've got this crater, this white section and it's got this blast zone where it's killed everything around it.
Chris - Yeah, so the bug colony from your desk is in the middle and there's nothing growing about a centimetre all round it. It's just killed off all the bugs.
Graihagh - I know, it's pretty amazing, isn't it? I'm kind of glad to have that on my keyboard. Maybe I'm protected againstE.colinow.
Chris - Maybe we should share our keyboards and rotate them round the office.
Graihagh - Well, you can see the pictures for yourself on facebook. Just head to facebook.com/thenakedscientists.

38:32 - How do antibiotics work?
How do antibiotics work?
with Georgia Mills & Tom O'Hanlon, Naked Scientists
如何抗生素,包括uding those being made on my keyboard, actually work? Here's Georgia Mills and Tom O'Hanlon with the gruesome ways in which antibiotics annihilate bacteria...
work? Here's Georgia Mills and Tom O'Hanlon with the gruesome ways in which antibiotics annihilate bacteria...
Georgia - There are five main ways that antibiotics kill bacteria, and they all involve exploiting differences between the structures of bacterial and human cells:
Tom - The first drug on the market was penicillin, discovered by Alexander Fleming back in 1929. This blocks the ability of the bacterium to build a tough outer cell wall; this causes it to swell up and burst...
Georgia - Second, below the cell wall is the cell membrane; this regulates concentrations of salts and water inside the cell. Antibiotics called Polymyxins break open the membrane, causing the bug to spill its guts and die.
Tom - Thirdly, bacteria use DNA, just like we do. And antibiotics such as ciprofloxacin, which is sometimes given to patients with urine, skin and chest infections, stop bacteria from copying or repairing their DNA, causing them to keel over.
Georgia - Fourth, you can stop a bacterium in its tracks by ensuring it can no longer make the vital nutrients it needs to live. For instance, sulphonamide antibiotics, like trimethoprim, prevent bugs from making the essential folic acid that they need to grow.
Tom - And finally, number 5, you can send in a drug that stops the assembly line in a bacterium's protein factory. Without proteins a cell can't survive. Drugs like tetracycline work this way and are commonly prescribed for people with acne or chest infections.
Georgia -So, in summary, to kill a bug you can burst it, pop it, neuter it, starve it or just break it...

40:11 - How bugs become resistant to the drugs
How bugs become resistant to the drugs
with Dr Hendrik van Veen, University of Cambridge
How do bugs sidestep antibiotic assassination? Hendrick van Veen from the University of Cambridge works on how this happens and he told Chris Smith about one mechanism called an efflux pump...
University of Cambridge works on how this happens and he told Chris Smith about one mechanism called an efflux pump...
Hendrick - Efflux pumps are really interesting ways by which bacteria can overcome toxicity of antibiotics.
Chris - Might one consider these efflux pumps to be a little bit like a bacterial vacuum cleaner, which sits in the cell and grabs stuff; detritus, dirt, molecules that shouldn't be there and just picks them up and throws them out?
亨德里克-是的。所以抗生素是化合物can bind to membranes and then it can move into cells and bind on the inside of the membrane and from there they move on to bind to targets. And theses efflux pumps they are in the membrane and have binding sites that basically suck up antibiotics from the membrane and then throw it out.
Chris - So they sound like quite bad news if they're able to spread and propagate. Why can't we just make some molecules which are, effectively, antibiotics which will go in there and block these efflux pumps up?
Hendrick - Well, one of the reasons is we in our body also have multidrug efflux pumps and they're really important for the protection of organs, like the brain against toxic compounds. And so if we were able to make inhibitors of bacterial efflux pumps, there is a very good chance that these inhibitors will also block efflux pumps in our body.
Chris - Oh dear. So the whole premise of an antibiotic chemical which is exploiting differences between bacterial cells and human cells, that would be lost because if they are so similar to our own, then we end up causing harm to ourselves in the process of trying to kill the bug?
Hendrick - Indeed. That has been the strategy so far. I think these days people look in very great detail at the shape of these efflux pumps and from the structures, it might be possible to make new agents that are really selective for bacterial efflux pumps and not for the human ones.
Chris - So how do bacteria become resistant other than by having efflux pumps?
Hendrick - One important mechanism is a mechanism by which bacteria can modify the antibiotic of maybe degrade the antibiotic.
Chris - They could just break it down. How else?
Hendrick - You also have mechanisms where you have mutations in targets that actually may no longer interact with the antibiotic.
Chris - So in effect changing shape so the antibiotic molecule can no longer dock with whatever it was hitting before?
Hendrick - Yes. And also, sometimes, you have amazing mechanisms where bacteria produce a lot of targets and then some of these targets escape the actions of antibiotics. And then the pathways that normally lead to the synthesis of new building blocks for new cells still occur.
Chris - So basically they're just outcompeting the antibiotic because they make so much of the thing the antibiotic hits that there's just no way of getting enough antibiotic in to outcompete what the bugs doing?
Hendrick - Yes, indeed. I think it really shows that antibiotic resistance mechanisms co-evolved with the use of antibiotics as a way to compete with other organisms in the natural environment.

43:15 - Giving germs a cold of their own
Giving germs a cold of their own
with Professor Martha Clokie, University of Leicester
Part of the problem of antibiotic resistance lies in the fact that these bacteria evolve resistance to our drugs. But what if you could have an antimicrobial agent that evolves with the bacterium so that, no matter how many clever tactics the bacterium develops, the treatment will stay in step and keep on killing it? This is what the University of Leicester's Martha Clokie has been working on. She's using viruses that selectively attack bacteria - these are called bacteriophages - to tackle hospital superbugs and she told Graihagh Jackson about her work...
Martha - Bacteria have got natural viruses that infect them. So in the same way that we have, for example, flu and ebola viruses that attack humans, bacteria have got their own natural enemies in the form of viruses that target and kill them.
Graihagh - How do they do that?
Martha - They attach and then they inject their DNA into that bacterial cell. They turn that bacterial essentially into a virus factory and then they pop out and maybe a hundred viruses will be released and then specifically go and infect other members of that same bacterial species.
Graihagh - Sounds like a lovely way to die. Why is this virus better than using antibiotics then?
Martha - Well, as I said, viruses are also natural enemies of bacteria. Your guest, he was talking about how antibiotics are part of this bacteria-bacteria warfare, and that's really been fairly well characterised. But this interaction between the viruses is much less known. And one real advantage that the viruses have, which in a way makes them quite difficult to study is that they're very, very specific. As you said, I'm working with superbugs, Clostridium difficile, and viruses that will infect them won't infect other bacteria that are in us and on us.
Graihagh - I know there are multiple strains of things like C. diff, so would you need one virus to kill them all? How specific are you talking?
Martha - Yeah. So they're specific within a species and also within a subset. So with Clostridium difficile there's over 450 different types. But generally, if you look at any one hospital at a time you will find there's perhaps twelve strains circulating. Just four viruses, actually, within Clostridium difficile are enough to be able to kill 90 percent of all of the strains that are commonly found, so you do need a number of viruses.
Graihagh - That's pretty good going. So the other thing I know that's really important about when you're using a virus is biofilms, the things that bacteria create to stick to where we get infected, and I know that your viruses are actually taking away that. But what's the benefit of this technique?
玛莎-一个是他们特定的事实。If you take a virus, if you've got that hospital superbug, Clostridium difficile, and I give you a set of viruses, I will just remove that one species, not the others. So often, when we take antibiotics, they make us feel very groggy because they kill lots of bacteria that are performing useful functions on us. So it's a sort of selective sharpshooter, the removal of that one pathogen.
Graihagh - And how do you envisage just taking this - like a pill?
Martha - Well, yes, exactly. I'm working with my collaborators at the University of Loughborough and we've shown that we can encapsulate these viruses into a pH-sensitive polymer so we can take them the same as you take an antibiotic. So you'd take this pill full of viruses and then get through that acidic stomach, because viruses don't survive well in those acidic conditions, so you've got to get them through the stomach into the area where Clostridium is causing the infection.
Graihagh - And there's no danger of us being infected by this virus as well, is there?
Martha - No, absolutely not. The viruses need very specific proteins on the surface of the receptors to hook onto so there's no way that they could jump from a bacteria to a human. Even, as I say, within the bacterial species they're pretty selective.
Graihagh - Awesome! So Martha, you were on the show three years ago - how has your research progressed since then?
Martha - Yes, it gone really well. I think about three years ago, we had a set of viruses and I was able to tell you yes, our viruses look like they kill the right kind of thing. And now I can tell you yes, we only need four and it's useful having these four because they kill in different ways. And the advantage of that is that we really minimise resistance.
The other thing that we've done is designed lots of different complicated models to mimic how the virus kills us. So we can create them on artificial guts and epithelial cells, and whole bunch of different, more complicated insect and other models and we've shown that the viruses work really well in those different models.
Graihagh - That sounds really exciting. Very briefly, you know the question I'm going to ask you. When are we going to be seeing this in the clinic or is it there already?
Martha - Well, there are clinical trials that are currently going on with other drugs at the moment. There's an EU project that's doing a study and there's another one in the States and Australia. So we're seeing clinical trials now for the first time as a revival of those. You can have them in the clinic in some countries, Russia or Georgia, but we're cottoning on to the fact that we need to use them in the West and clinical trials are finally starting to happen.

48:17 - How worried should we be?
How worried should we be?
with Dr Nick Brown, Addenbrooke's Hospital
A report in 2014 estimated that 700,000 people a year die from antibiotic 抗感染,如果不采取措施number could rise to 10 million a year by 2050. Dr Nick Brown a medical consultant from Addenbrooke's Hospital and he told Chris Smith about what could happen if we don't find a solution...
抗感染,如果不采取措施number could rise to 10 million a year by 2050. Dr Nick Brown a medical consultant from Addenbrooke's Hospital and he told Chris Smith about what could happen if we don't find a solution...
Nick - I think although, as you say, these are only estimates, the numbers are really quite scary. The same report that you quoted also put a financial value on antimicrobial resistant and estimated that by 2050 the cost to the world would be in the order of 66 trillion dollars, These are really quite scary numbers.
Chris - Is this lives lost, productivity lost, infection control mechanisms in hospital - is that where the money's going?
Nick - All of the above really. So, just to give two examples, if we don't have antibiotics to treat infections in patients with cancer who are having chemotherapy, then we might not be able to treat the infections that these patients have and they may not survive their treatment.
Likewise, if we can't do hip replacements without patients getting infections that can be treated, then why do the hip replacement, and then people can't go to work. So all sorts of effects and these estimate try to take all those into account.
Chris - We made antibiotics in the first place. We've had plenty so far, it's only now become a problem when we're better technologically endowed than we have ever been. So why don't we just make new antibiotic molecules?
Nick - There really has been a dearth of new antibiotic development in the last twenty years or so, and think this has really crept up on us. We're really used to simply replacing an antibiotic with another one when we can't use it any more because of resistance. but now there just aren't any left. It takes a long time to develop an antibiotic and, in recent years, a lot of the big pharmaceutical companies have decided that this really isn't an economic field or market that they want to be in.
Chris - Is that because there's a real risk your drug is going to quickly develop resistance, therefore you might not make your money back so much better to go for conditions like high blood pressure, stomach ulcers because people will be on your tablets for years and you'll make lots of money?
Nick, Absolutely, yeah.
Chris - Shouldn't the government step in then?
Nick - It's something that is being debated at the moment internationally. Is antibiotic resistance a public health issue, not something that should be governed by a market?
Chris - Now why is this happening at all in the sense that if you look at the pre-antibiotic era, before we knew about antibiotics, before we even knew that bacteria caused things like tuberculosis, death rates in London, for example, in the 1800s were plummeting? People were living much longer and they weren't dying of these infections, so why is this happening now, can't we just live a cleaner life?
尼克,我同意你的观点。我认为生活蜡烛aner life helps prevent infection. I think improved hygiene is an integral part to the control of antibiotic resistance. If we look to developing countries where some of the hygiene standards available, sewers, clean drinking water, etc. is not so good, then these are hotbeds for the spread of antibiotic resistance because the prevalence of infection in these areas is so high. We do have to do something worldwide to improve the situation and that will help us not control, but to keep antibiotics sustainable into the future. I think we'll need to learn how to use or treat antibiotics as a scarce resource.
Chris - China aren't doing that are they? The Lancet published a paper last year showing twelve and half thousand tons of colistin, an antibiotic of last resort here in the UK for certain infections, and they give it to farm animals.
Nick - Which is why it is so important that we emphasise that this is a true what is called a "one health" issue. That it's not just antibiotic use in humans but also in agriculture and in the environment as well.

52:21 - How YOU can stop antibiotic resistance
How YOU can stop antibiotic resistance
with Dr Nick Brown, Addenbrookes Hospital
Your prescription to help stop antibiotic resistance with Nick Brown and Graihagh Jackson, beginning with why you should finish your course of antibiotics...
Nick - It's quite possible that the infection that you had will either come back or relapse in some way and need further antibiotics in the future. And, unfortunately, because resistance can develop within you, as well as within the population of bacteria, it's quite possible that the next time round you won't respond to the antibiotic that you're given.
Graihagh - Number 2 - don't use your friends, presumably this is because you might not be treating the correct infection and I guess that's bad for exactly the same reasons that you've just stated?
Nick - Yes. And I think it's very important to stress that people shouldn't access antibiotics themselves. There are a number of ways that you can do that - some legal and some not so legal.
Graihagh - like on the internet maybe?
Nick - Absolutely yes. And you should always access antibiotics through a registered prescriber.
Graihagh - I didn't even know you could order antibiotics on the internet actually!
Number 4 - Don't pressure your doctor for antibiotics for a cold? I know that's a virus now after this show but is it just that you're making your doctor feel pressured and, I suppose, the other side of that is that's not very nice really?
Nick - Yes, and I think it's important that people know what to expect from a viral infection as well, not just that viruses don't respond to antibiotics. But, for example, if you have a sore throat, you may still have symptoms six or seven days after it started. If you have bronchitis, it can go on for three weeks, which is really quite a long time and, if you don't appreciate that, then you may be worried and go to your doctor expecting antibiotics. It's important to get proper advice if you are failing to get better, but if you know what to expect in various situations, then perhaps you'd be happier to go to a pharmacy.
Graihagh - So trust your doctor.
And lastly, number 5 - wash your hands. I know this is pretty obvious given what we've just been talking about, but how long for? I've heard it's as long as you can sing happy birthday - is that right?
Nick - Or yankee doodle is the other one. Yes, probably longer than we all do it at the moment is the easy answer, perhaps. But certainly, washing your hands is one of the most effective ways that you can stop any infection from being transferred from one person to another.
Graihagh - And hand sanitiser versus soap?
Nick - What we do in hospitals is say that if you hands are physically clean, that is they're not covered in dirt, then an alcohol sanitiser is absolutely fine. If they're dirty you do need to wash them because, unfortunately, alcohol will fix dirt onto your hands.
Graihagh - Um - lovely!
Chris - And the other thing you need to bear in mind of course is that lots of these viruses that live on your skin are not vulnerable to alcohol so you end up with a pure culture of norovirus, or rhinovirus or whatever else. So you should just wash your hands.

55:40 - Why doesn't water burn?
Why doesn't water burn?
Water contains hydrogen and oxygen, both of which are important in combustion, so why is it used to put out fires? Why doesn't water burn? Graihagh Jackson put this to chemist Peter Wothers from the University of Cambridge...



Comments
Add a comment